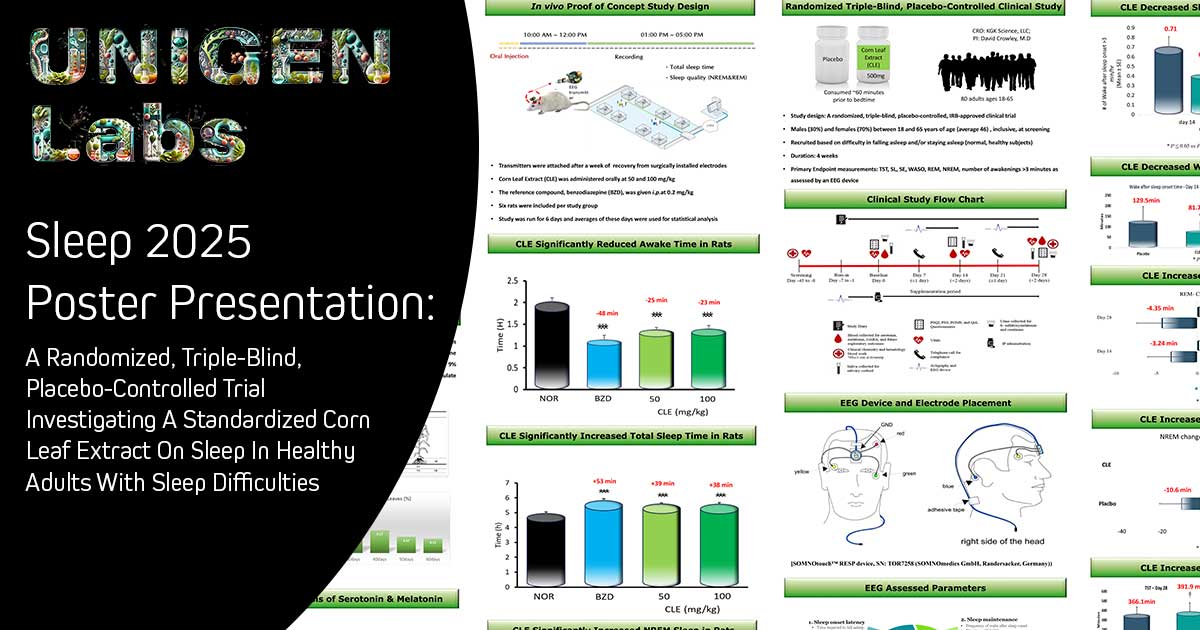
Executive Leadership

Regan Miles
President & CEO
 Mr. Miles began his career with Unigen in 2002 as EVP of Sales & Marketing. He has served two terms as Unigen’s President & CEO, from 2006-2008 and 2015 to present. During his time with Unigen, Mr. Miles has been instrumental in growing the business from a new research and development firm to a profitable patented ingredient supplier. Under his leadership the biotech company launched 10 proprietary branded ingredients that ultimately brought to fruition the vision and mission of “Bringing the best of nature to humankind.”
Mr. Miles began his career with Unigen in 2002 as EVP of Sales & Marketing. He has served two terms as Unigen’s President & CEO, from 2006-2008 and 2015 to present. During his time with Unigen, Mr. Miles has been instrumental in growing the business from a new research and development firm to a profitable patented ingredient supplier. Under his leadership the biotech company launched 10 proprietary branded ingredients that ultimately brought to fruition the vision and mission of “Bringing the best of nature to humankind.”
Mr. Miles has 30+ years of experience in the natural products industry. He has held top leadership positions with many of the leading companies in the industry including Nature’s Way, Pharmanex, Integrative Therapeutics, Univera and Next Pharmaceuticals. He has demonstrated a strong record of success in achieving growth and profitability at the companies he has been associated with.
Qi Jia
Chief Scientific Officer
 Dr. Jia has worked for the company nearly 30 years since Unigen’s inception in 1996. Since joining Unigen as Director of Natural Products in 1996, Dr. Jia has established collaborations with multiple organizations around the world to collect indigenous medicinal plants and has built up a proprietary natural products library and the discovery platform, PhytoLogix®, with thousands of collections. He has led product development and commercialization of nutritional and cosmetic ingredients with expanded responsibility as Vice President of Product Development between 1999 – 2004 and as Chief Scientific Officer from 2004 to present. Dr. Jia holds 107 issued international patents and has 73 peer-reviewed publications and 64 oral or poster presentations at professional conferences in the field of natural products research. The ingredients Univestin® and Nivitol®, co-invented by Dr. Jia and Unigen scientists, have been utilized by global brands in joint health and even skin tone products. Dr. Jia received his B.S. in Medicinal Chemistry from Beijing Medical University in 1985 and a Ph.D. in Organic Chemistry from Texas Christian University in 1995. Dr. Jia has studied ethnomedicinal plants for over 35 years. He has demonstrated a strong leadership and record of success in natural products discovery, development, commercialization and establishment of R&D collaborations with universities, institutes and industry partners.
Dr. Jia has worked for the company nearly 30 years since Unigen’s inception in 1996. Since joining Unigen as Director of Natural Products in 1996, Dr. Jia has established collaborations with multiple organizations around the world to collect indigenous medicinal plants and has built up a proprietary natural products library and the discovery platform, PhytoLogix®, with thousands of collections. He has led product development and commercialization of nutritional and cosmetic ingredients with expanded responsibility as Vice President of Product Development between 1999 – 2004 and as Chief Scientific Officer from 2004 to present. Dr. Jia holds 107 issued international patents and has 73 peer-reviewed publications and 64 oral or poster presentations at professional conferences in the field of natural products research. The ingredients Univestin® and Nivitol®, co-invented by Dr. Jia and Unigen scientists, have been utilized by global brands in joint health and even skin tone products. Dr. Jia received his B.S. in Medicinal Chemistry from Beijing Medical University in 1985 and a Ph.D. in Organic Chemistry from Texas Christian University in 1995. Dr. Jia has studied ethnomedicinal plants for over 35 years. He has demonstrated a strong leadership and record of success in natural products discovery, development, commercialization and establishment of R&D collaborations with universities, institutes and industry partners.
Rodney E. Storms, JD
Executive Sales Director
 Rodney E. Storms was named Executive Sales Director, Sales & Marketing, Unigen, Inc., effective June 1, 2024.
Rodney E. Storms was named Executive Sales Director, Sales & Marketing, Unigen, Inc., effective June 1, 2024.
Mr. Storms joined Unigen as Regional Sales Manager in 2005, served as Director of Regional Sales from 2016-2021 and more recently as Vice President, Sales & Marketing, from 2021-2024. He has been very instrumental in establishing key customer accounts for Unigen’s extensive pipeline of novel cosmetic, nutra and pet ingredients. In addition, Mr. Storms helped establish several clinical collaborations with potential customers, which saved Unigen large financial investments, and created immediate customers. Mr. Storms is instrumental in bringing in many PhytoLogix® deals over the years, helping to establish our Plant Library Platform as one of the best globally.
Mr. Storms received his B.A. in Political Science and B.A. in Sociology from The University of Texas at San Antonio. He followed his undergraduate work with a Juris Doctorate at The Columbus School of Law, Catholic University of America. Prior to joining Unigen, Mr. Storms was the Director of Membership and Interim President, American Herbal Products Association (AHPA).
As Executive Sales Director, Mr. Storms will lead Unigen’s Sales & Marketing team through substantial growth and diversification via products, customers, channel of business and continue to fulfill its global vision of “Bringing the best of nature to humankind.”
Mesfin Yimam, DVM, MS.
Director, Pre-Clinical Research
 Dr. Mesfin Yimam joined Unigen in December 2004, with practical experience in pharmaceutical research and veterinary medicine. Dr. Yimam is a board certified DVM. He received his doctorate degree in veterinary medicine from Addis Ababa University, Ethiopia. He further broadened his knowledge in the field of drug discovery by studying pharmaceutics and has acquired his master’s degree at the University of Washington in Seattle, where he focused on identifying and characterizing primate P-gp and illustrating target specific drug delivery in syngeneic experimental brain tumor model.
Dr. Mesfin Yimam joined Unigen in December 2004, with practical experience in pharmaceutical research and veterinary medicine. Dr. Yimam is a board certified DVM. He received his doctorate degree in veterinary medicine from Addis Ababa University, Ethiopia. He further broadened his knowledge in the field of drug discovery by studying pharmaceutics and has acquired his master’s degree at the University of Washington in Seattle, where he focused on identifying and characterizing primate P-gp and illustrating target specific drug delivery in syngeneic experimental brain tumor model.
Dr. Yimam currently holds a director position leading the Pre-Clinical Development team at Unigen. His research interest is primarily focused on the discovery of natural products based novel dietary ingredients for osteoarthritis, metabolic disorders, sleep, brain health and immunity. Over the years, he has made significant contribution in multiple projects through development and validation of several in vivo disease models; optimization of in vivo experiments to support safety, efficacy, mechanism of actions and pharmacokinetics studies, ensuring research strategy and procedures in compliance with regulations and organization guidelines pursuant to FDA and other regulatory agencies. Dr. Yimam has published more than 40 peer reviewed articles, co-invented several issued and pending patents, presented his work in a range of scientific conferences and is also an editorial board member and invited reviewer for reputable journals for scientific publications.