What Makes Univestin® Unique?
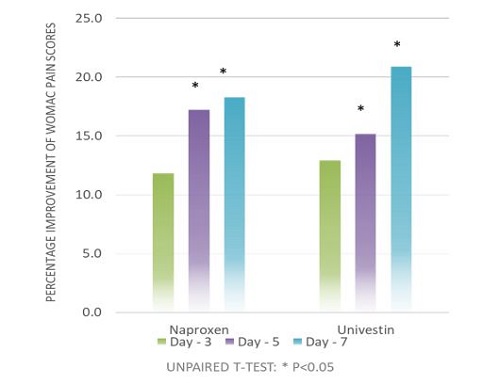
- Clinically shown to promote joint flexibility within 3 days*
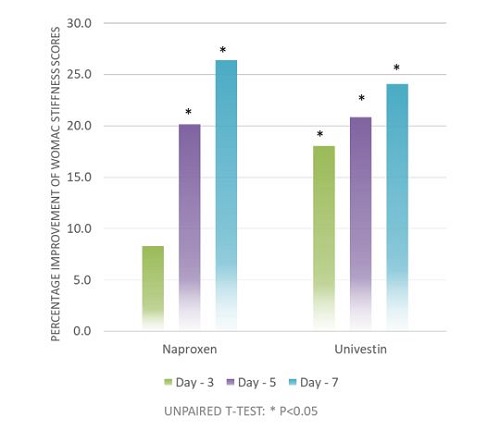
- Clinically shown to promote joint comfort within 5 days*
- Clinically shown to support range of motion and physical function within 7 days*
- Backed by two randomized, double-blind human clinical trials
- Proven safe through dozens of safety studies and long-term human consumption
- Provides potent antioxidant activity*
- Promotes healthy aging and overall wellbeing*
Univestin® Key Benefits*
- Clinically proven to provide rapid and long lasting relief from joint discomfort.
- Improves range of motion, flexibility and physical function.
- Natural substances with history of safe human use.
- Extensive in vivo and in vitro safety testing with no adverse effects.
- High compatibility in formulating with other ingredients.
Plant Origin
Derived from Scutellaria baicalensis and Acacia catechuApplications
- Alleviate joint discomfort and stiffness.
- Improve mobility and range of motion.
- Enhance flexibility and physical function.
Formulation
Can be used as an active agent in tablets, capsules, liquids, powders, bars and other delivery systemsPhysical Properties
Greenish yellow to brown colored powder that partially dissolves in water. *Indications and claims related to the health benefits or property of an ingredient or product are intended for industry only and are governed in accordance with country-specific laws and regulations and may not be appropriate for final consumer products. In the United States, it is your responsibility to ensure that product claims and indications are in compliance with all applicable laws and regulations, including the Federal FD&C Act and the FTC Act. In all other countries, please consult with a local regulatory or legal professional who may provide you with competent advice and guidance.Discovery
Univestin®’s unique formulation was developed through Unigen’s proprietary Phytologix® technology platform, based on a collection of more than 12,000 plants and marine samples with documented human consumption. Unigen scientists used this library of comprehensive botanical profiles to identify plants whose actives delivered the most effective joint health support in in vitro cell assays.*
Univestin® is a novel plant-based ingredient, derived from the roots of Scutellaria baicalensis and heartwood of Acacia catechu.
Over 1230 medicinal plants from Unigen’s PhytoLogix™ library were screened to identify natural substances with COX and LOX dual inhibitory activity. The Univestin® bioflavonoids, baicalin from Scutellaria baicalensis, and catechins from Acacia catechu (Senegalia catechu) were identified as the active components of the most effective extracts tested both in-vitro and in-vivo.
The combination of these two extracts produced a synergistic effect in-vivo with regards to both onset and duration.
Mechanism of Action
- Modulating parallel enzymatic pathways (COX and LOX) that result in increased joint health support*
- Increasing antioxidant activity, which neutralizes ROS production and oxidative stress*
- Helping regulate gene expression, which ramps up the body’s natural cartilage production process*
Scientific Research
Pre-Clinical studies
Extensive pre-clinical data has shown the beneficial effects of Univestin® related to supporting joint comfort. The bioflavonoid components of Univestin® were discovered as inhibitors of the pro-inflammatory COX and LOX enzymes. The IC50 for inhibition of COX-1 by Univestin® was calculated to be 0.2 µg/mL/unit of enzyme and the IC50 for inhibition of COX-2 was calculated to be 0.4 µg/mL/unit of enzyme. Scientific data demonstrated that Univestin® was also an effective inhibitor of the LOX pro-inflammatory pathway.
The IC50 of Univestin® for inhibition of the LOX pathway was 25 µg/mL in the LPS stimulated THP-1 cells. Chondrocytes and inflammatory cells, e.g., neutrophils and macrophages, produce high amounts of ROS such as superoxide anion, H2O2 and HO– that are causally linked to cartilage degradation.1,2
Studies demonstrate that Univestin® is a potent antioxidant having an oxygen radical absorbance capacity (ORAC) 36 times higher than that of the commonly used anti-oxidant Citrus Bioflavonoids.
Three experimental systems were utilized to evaluate Univestin® for regulation of pro-inflammatory gene expression: human peripheral blood monocytes (hPBMCs), the human monocytic cell line 28SC, and the mouse macrophage cell line RAW 264.7.
Univestin® significantly decreased pro-inflammatory Il-1β, IL-6, and COX-2 gene expression and TNF-𝛼 secretion in human PBMCs treated with lipopolysaccharide. Univestin® significantly decreased LPS-induced expression of TNF-𝛼, IL-1β and NFκb in human 28SC monocytic cells. Univestin® changed the expression of LPS-induced cytokines and chemokines in RAW 264.7. cells to more closely mimic untreated controls, as assessed by microarray.3
Clinical Studies
Two independent clinical research institutes performed two human clinical trials to demonstrate clinical efficacy of Univestin®.
A human clinical study with Univestin® was conducted at Florida State University (Tallahassee, Florida, United States) in 2011. The clinical trial was a single-center, randomized, double-blind, positive-controlled study. For this trial, a total of 80 subjects were randomized (40 per group), subjects were balanced by demographics, such as age, gender, and BMI.
The data was analyzed by an independent statistician and ANOVA techniques were used to assess effect of usage over time. The principal objective of this study was to examine the quick onset effects of a 1-week, daily supplementation with Univestin® at 500 mg/day on the indicators associated with OA as compared with a positive control – an NSAID drug (non-steroidal anti-inflammatory) with a dosage of 440 mg /day.4
The results suggested that Univestin® performed well with respect to the discomfort section of the WOMAC scale and faster benefit with respect to improving stiffness when taken for one week.
Additionally, the statistically significant increase in ROM (Range of Motion) within the Univestin® group from baseline to day 7 day suggests that Univestin® aids in joint flexibility and also supports increased comfort and mobility over one week of supplementation.
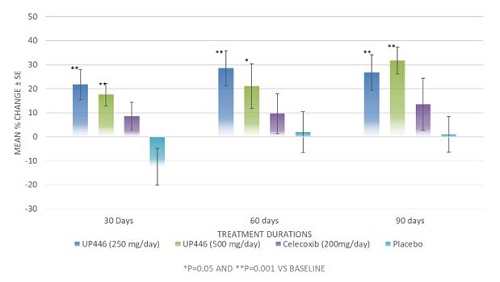
Univestin® has demonstrated significant results in joint mobility and function in another study conducted by JSS Medical Research Inc. (Montreal, Quebec, Canada) in 2003.5 The clinical trial was a single-center, randomized, double-blind, placebo and positive comparator-controlled study. Sixty subjects (n=60) with rheumatoid arthritis or osteoarthritis of the knee and/or hip were recruited for this study. Supplementation consisted of oral administration for 90 days of the placebo, Univestin®, or an active control NSAID drug (non-steroidal anti-inflammatory) according to the above dose schedule.
This study provided data to support that Univestin® at 250 mg/day and 500 mg/day was significantly effective for the support of joint mobility and joint function within 30 days (p = 0.010) of use and Univestin® 500 mg/day was effective for support of comfort at 30 days (p = 0.020).5

Product Safety
Acute toxicity, repeated chronic toxicity, safety pharmacology, reproductive and developmental toxicity and genotoxicity in in vivo safety studies have demonstrated the safety of Univestin®. Furthermore, extensive human clinical safety studies were conducted on Univestin® at multiple dose levels and different durations. Dosages from 250 mg to 1,100 mg per day were administered orally in capsules/tablets without significant adverse effect compared to placebo group.6
Univestin® has been proven to be safe for human consumption with more than 6 billion doses consumed at the recommended daily dosages.
References
- Tiku M.L., S. Gupta, D.R. Deshmukh. 1999. Aggrecan degradation in chondrocytes is mediated by reactive oxygen species and protected by antioxidants. Free Radic Res.30(5):395-405.
- Burnett BP, Q. Jia, Y. Zhao, R.M. Levy. 2007. A medicinal extract of Scutellaria baicalensis and Acacia catechu acts as a dual inhibitor of cyclooxygenase and5-lipoxygenase to reduce inflammation. J Med Food. 10(3):442-51.
- Tseng-Crank J., S. Sung, Q. Jia, Y. Zhao, B. Burnett, D. Park, S. Woo. 2010. A medicinal plant extract of Scutellaria Baicalensis and Acacia catechu reduced LPS-stimulated gene expression in immune cells: a comprehensive genomic study using QPCR, ELISA, and microarray. J Diet Suppl. 7(3). 253-72.
- Arjmandi B.H., L.T. Ormsbee, M.L. Elam, S.C. Campbell, N. Rahnama, M.E. Payton, K. Brummel-Smith, B.P. Daggy. 2014. A Combination of Scutellaria Baicalensis andAcacia Catechu Extracts for Short-Term Symptomatic Relief of Joint Discomfort Associated with Osteoarthritis of the Knee. J Med Food. 17 (6), 707–713.
- Sampalis J., L.A. Brownell. 2012. A randomized, double blind, placebo and active comparator controlled pilot study of UP446, a novel dual pathway inhibitor anti-inflammatory agent of botanical origin. Nutrition Journal. 11(21). doi:10.1186/1475-2891-11-21.6.Intertek Scientific & Regulatory Consultancy Health, Environmental & Regulatory Services. 2018. Safety Evaluation of Univestin®
Copyright © 2022 Unigen® All Rights Reserved. This information is provided for scientific and educational purposes related to this ingredient. When marketing this ingredient in a finished product to end consumers, it is your responsibility to ensure that product claims and indications are in compliance with all applicable laws and regulations including the Federal FD&C Act and the FTC Act
*These statements have not been evaluated by the Food & Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease. These statements are presented for informational purposes only and are not intended to be presented to final consumers.
Want to learn more about Univestin®?
"*" indicates required fields