Unigen showcased compelling clinical data supporting the sleep-enhancing properties of Maizinol® at the SLEEP 2025 conference in Seattle this June. Chief Science Officer Qi Jia, PhD, and Director of Pre-Clinical Research Mesfin Yimam, DVM, MS, presented the EEG clinical trial results in their poster titled “A Randomized, Triple-Blind, Placebo-Controlled Trial Investigating A Standardized Corn Leaf Extract On Sleep In Healthy Adults With Sleep Difficulties.”
Study Significance
This rigorous clinical investigation represents a significant advancement in natural sleep support research, utilizing objective EEG measurements to evaluate Maizinol®’s efficacy. The triple-blind, placebo-controlled design adheres to the highest scientific standards, providing robust evidence for the ingredient’s sleep-promoting benefits in adults experiencing sleep difficulties.
About SLEEP 2025
SLEEP 2025 marked the 39th annual meeting of the Associated Professional Sleep Societies (APSS), is a joint meeting of the American Academy of Sleep Medicine (AASM) and the Sleep Research Society (SRS). The SLEEP meeting provides evidence-based education to advance the science and clinical practice of sleep medicine, disseminates cutting-edge sleep and circadian research, promotes the translation of basic science into clinical practice, and fosters the future of the field by providing career development opportunities at all levels.
Maizinol® EEG Clinical Results Poster
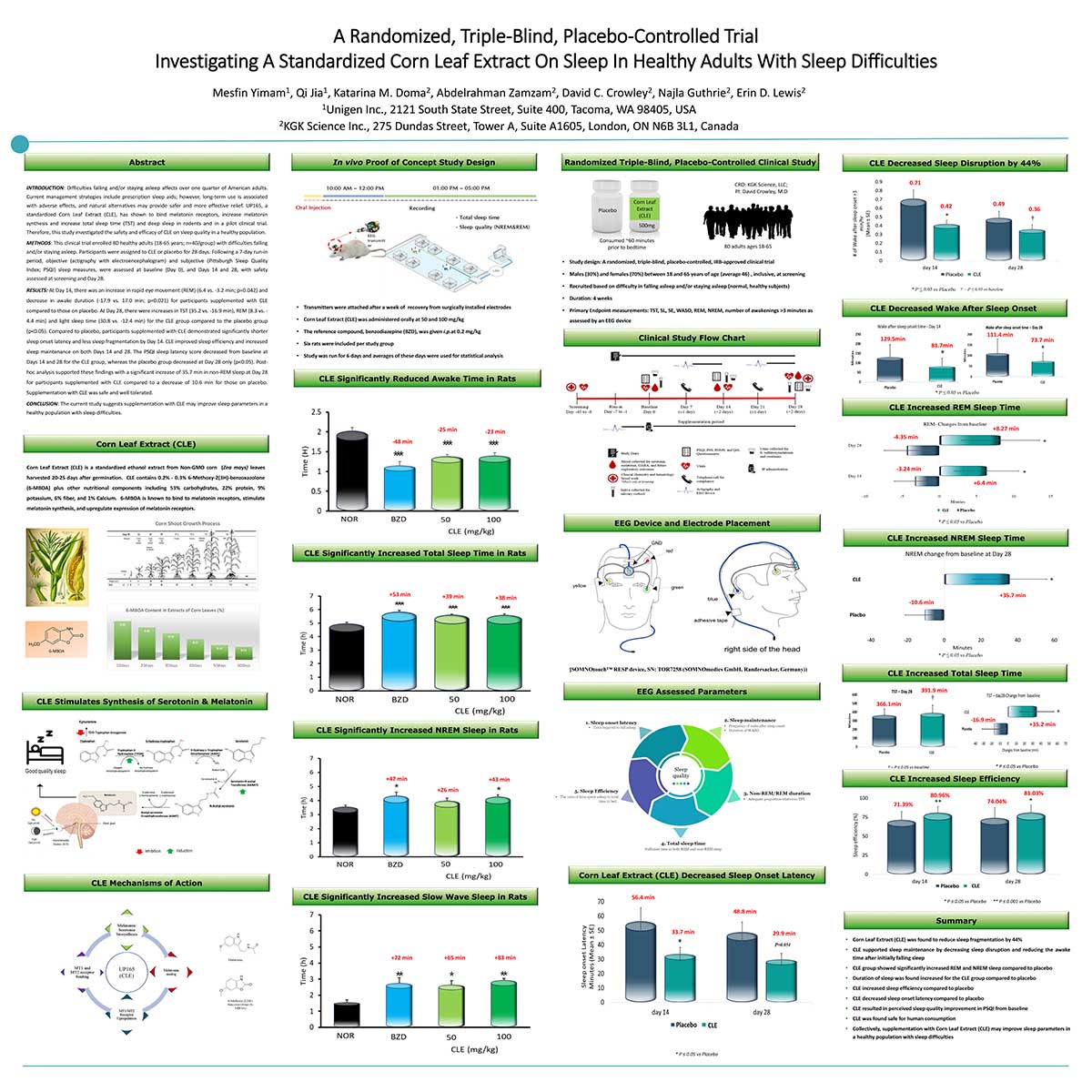
“A Randomized, Triple-Blind, Placebo-Controlled Trial Investigating A Standardized Corn Leaf Extract On Sleep In Healthy Adults With Sleep Difficulties“
by Qi Jia, PHd and Mesfin Yimam, DVM, MS.
Interested in learning more about the science behind Maizinol®? ➤ View the ingredient page